
Biocidal Products: 2024 Fee Rates in Turkey
Biocidal product fee rates for the year 2024 have been published.
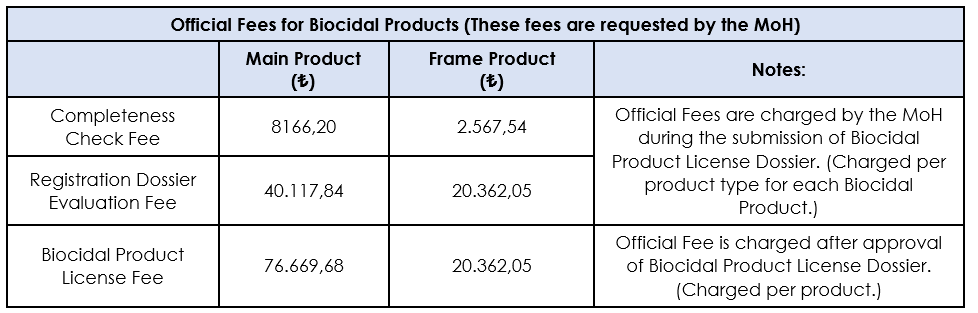
The current fee rates are listed in the "2024 Institution Services Price Tariff" published by the Turkish Ministry of Health, the Pharmaceuticals and Medical Devices Agency. According to this list, the fee for the Completeness Check for the Main Product is 8,166.20 TL and for the Frame Product is 2,567.54 TL. For Registration Dossier Evaluation processes, these fees are 40,114.84 TL and 20,362.05 TL, respectively, and they must be paid separately for each product type in license applications, and the payment receipt must be added to the relevant License application dossier. Finally, for the Biocidal Product License, the fee for the Main Product is 76,669.68 TL, while the fee for the Frame Product is 20,362.05 TL. These fees are paid per product after the approval of the license dossier for the issuance of the license. However, it should be noted that these published fees apply only to applications of biocidal products within the scope of Product Type 1 and Product Type 19 within TITCK.
You can click here to review the "2024 Institution Services Price Tariff," which includes other fees for various matters such as Drug License Certificate, License Renewal Application, and Advanced Treatment Medical Product Classification Application.
