The Cosmetic Product Safety Assessment (CPSR) is one of the fundamental elements of the Product Information File, which is mandatory for all cosmetic products intended for marketing within the EU to comply with Regulation (EC) No 1223/2009. As a result of this process, a Cosmetic Product Safety Assessment Report (CPSR) consisting of two parts is prepared.
Regulation (EC) No 1223/2009, Article 10 (1) states that before placing a cosmetic product on the market, the responsible person shall ensure that the cosmetic product has undergone a safety assessment based on the relevant information and that a cosmetic product safety report in accordance with Annex I is drawn up.
A CPSR must be prepared for all cosmetic products that come into contact with the skin and are intended for public sale.
The Cosmetic Product Safety Assessment Report serves as scientific evidence that a product will not cause harm under normal and foreseeable conditions of use. This ensures that products can be legally placed on the European Union market while ensuring consumer safety.
According to the EU Cosmetics Regulation, the cosmetic product safety assessment must be carried out by a person who has a diploma or formal qualification in pharmacy, toxicology, medicine, or a similar discipline obtained through the completion of a university course providing theoretical and practical training, or by someone who has completed a course recognized as equivalent by a member state.
The steps for preparing CPSR are as follows:
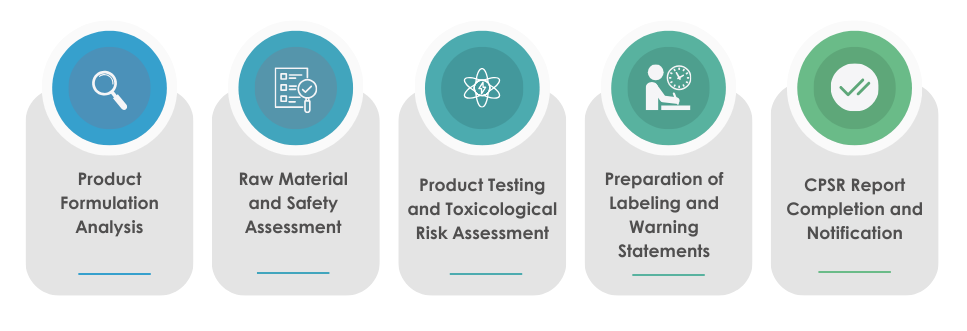
For cosmetic companies, the CPSR compliance is not just a regulatory requirement but also an assurance to their customers that they provide safe products.
Chemleg Consultancy can prepare a comprehensive Cosmetic Product Safety Assessment Report in compliance with Regulation (EC) No 1223/2009 with the help of its qualified experts.
Contact us today to learn more about our cosmetic product safety assessment report service and how we can help you achieve your business goals.
Click here to visit the contact page.