
PCN – Poison Centre Notification Obligations
What is the Poison Centre Notification – PCN Notification?
The Poison Centre Notification is one of the obligations established by ECHA for importers and downstream users introducing hazardous mixtures to the EU and European Economic Area (EEA) markets.
By notifying poison centres in the relevant countries of these mixtures, healthcare professionals who treat patients can quickly access accurate information about the substances exposed in the event of an accident. This enables more effective treatment for patients.
The Poison Centre Notification obligation in Europe falls under the EU Chemical Regulations and is stipulated as follows in Article 45 of the CLP Regulation:
“Article 45
Appointment of bodies responsible for receiving information relating to emergency health response
- Member States shall appoint a body or bodies responsible for receiving information relevant, in particular, for formulating preventative and curative measures, in particular in the event of emergency health response, from importers and downstream users placing mixtures on the market. This information shall include the chemical composition of mixtures placed on the market and classified as hazardous on the basis of their health or physical effects, including the chemical identity of substances in mixtures for which a request for use of an alternative chemical name has been accepted by the Agency, in accordance with Article 24."
What is the Deadline for the Poison Centre Notification?
Previously, it was stated that, as of January 1, 2024, importers and downstream users related to the EU and EEA are required to complete the Poison Centre Notification processes in accordance with the requirements set forth in Annex VIII of the CLP Regulation. For substances previously notified through national application systems and already on the market, a transition period has been established, which will end on January 1, 2025.
You can click here for ECHA's news on the subject.
Who Should Submit a Poison Centre Notification?
Importers, distributors (labelers, rebranders, and retailers), and downstream users involved in the production and distribution of hazardous mixtures are obligated to submit poison centre notifications. These roles are detailed in the chart below:
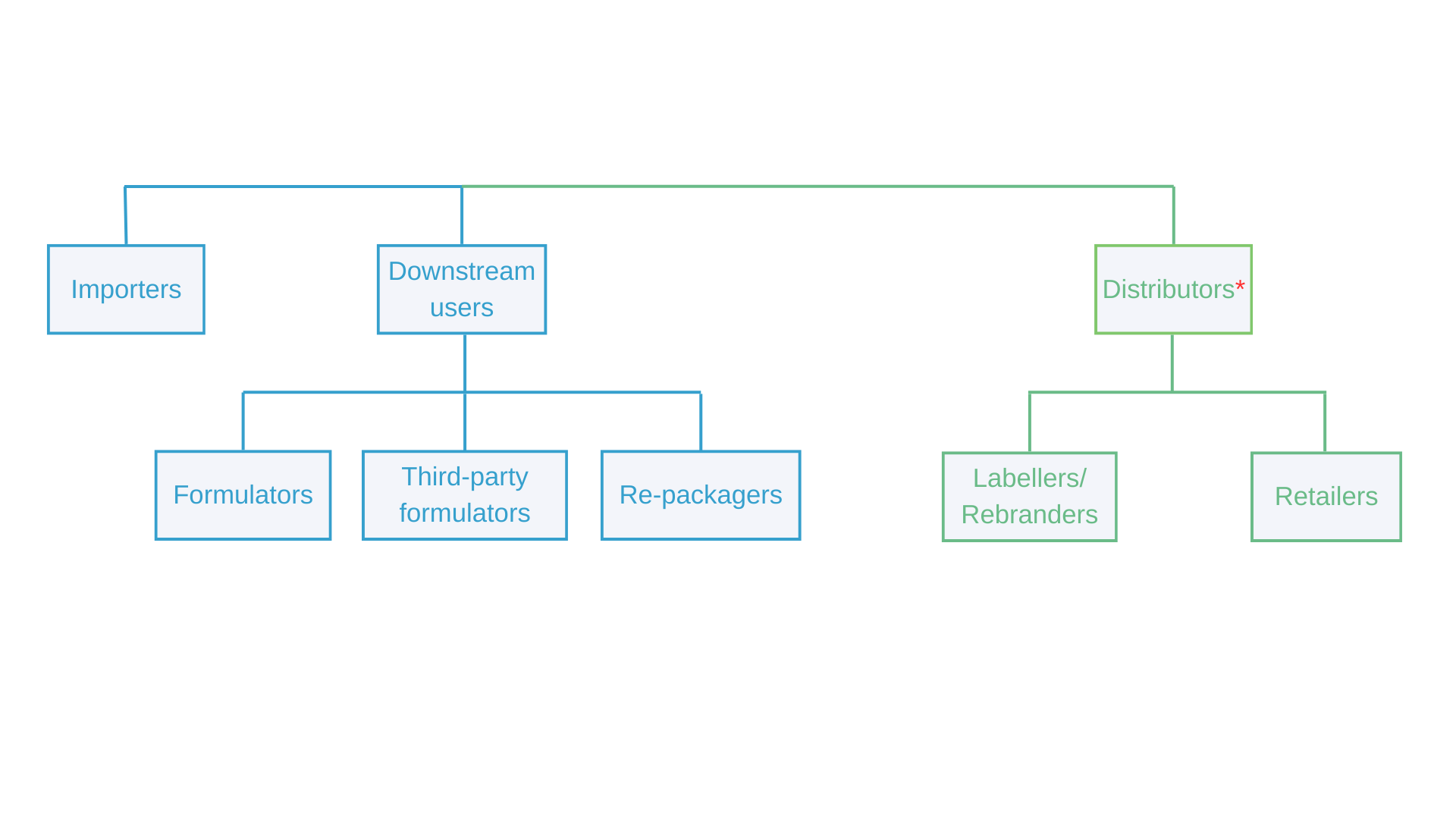
- CLP Regulation, Article 4 (10): Mixtures can only be placed on the market if they comply with the CLP Regulation, including for distributors.
In the Draft Amendment of CLP Regulation Article 45, the following statement is included:
“Distributors placing on the market mixtures that are classified as hazardous on the basis of their health effects or physical effects, shall submit to the appointed body or bodies the harmonised information referred to in Part B of Annex VIII where they further distribute those mixtures in other Member States, or where they rebrand or relabel the mixtures. This obligation does not apply if the distributors can demonstrate that the appointed body or bodies already received the same information from importers or downstream users.”
Distributors have two options regarding poison centre notification: either to submit a notification or inform their supplier. Distributors may prepare the PCN dossier and send it to the relevant member country or provide new name or target market information to their supplier.
How to Prepare a PCN Dossier?
The PCN dossier must contain the following information:
- Identification of the notifying company
- Product identification (trade names, UFI)
- Product classification and labeling elements
- Toxicological information related to the product
- Additional product information (physical state, color, pH, packaging, EuPCS codes, intended product use, final use of the product (consumer, professional, industrial)
- Composition details of the mixture (substances and/or mixtures-in-mixtures (MiMs))
It is essential that this information is added to the PCN dossier accurately and completely. If there is any change in this information, the dossier must be updated. Once prepared, the notification is made through ECHA. A more detailed explanation of the process is provided in the table below:
|
Step |
Process Title |
Required Information |
Details |
|
1 |
Classification of the Mixture |
Chemical Classifications, H-statements, Pictograms |
The chemical must be classified as hazardous according to the CLP (Classification, Labeling, and Packaging) Regulation. |
|
2 |
Creation of the UFI Code |
UFI (Unique Formula Identifier) |
UFI, the unique identification code of the chemical formula, should be generated via ECHA’s UFI creation tool or relevant software such as EPY UFI. |
|
3 |
Product Category Information |
Product type, application area |
The product category should be determined and specified using the European Product Categorisation System (EuPCS). |
|
4 |
Preparation of Component Details |
CAS Numbers, EC Numbers, Substance Concentrations |
Detailed component information and concentration percentages of all substances used in biocidal products should be prepared. This information may also be found in the REACH-compliant Safety Data Sheet (SDS). |
|
5 |
Entry of Product and Label Information |
Trade Name, Packaging Information, Color and Form |
All identifier information on the product label (e.g., trade name, package size) and the physical appearance of the product (colour, form) should be specified. |
|
6 |
Addition of Importer or Manufacturer Information |
Company Name, Contact Information |
The notification is made on behalf of the importing or manufacturing company in Europe; company information and contact details must be entered. |
|
7 |
Preparation of Use Scenarios |
Consumer or Industrial Use |
How and by whom the chemical will be used should be specified. Specific uses, safety precautions, or exposure scenarios should be added. |
|
8 |
Preparation and Submission of the PCN Dossier |
Creating a dossier in XML format |
The prepared information is converted into an XML file in the PCN format using ECHA’s IUCLID or similar tool and submitted to the Ministry of Health. |
What Awaits Companies that Do Not Complete the Notification by January 1, 2025?
The deadline for completing the Poison Centre Notification is approaching. If you have not yet completed the necessary notification or updated your dossier, as of January 1, 2025, you may face administrative fines, suspension or cancellation of activities, market loss risks, insurance issues, and damage to your company’s reputation. In such a situation, continuing your international trade smoothly will not be possible. So, how can you complete the necessary procedures quickly?
To ensure your PCN notification processes are carried out accurately, you can seek help from consulting firms, or to expedite the process, use supportive software like EPY Plus to prevent your company from encountering potential adverse situations.
With the EPY Plus PCN Module, you can easily submit your chemical product dossiers in the PCN format required by CLP Annex VIII to ECHA’s Poison Centre Portal.
For detailed information about the module, please contact our team.
