
Cosmetic Allergens and New Regulations | Turkey
Cosmetic Allergens and New Regulations
In 2003, the European Parliament issued Directive 2003/15/EC, which included a list of 26 allergens (Initially, there were 26 allergens, but two were banned: Lilial, Lyral).
In December 2011, the European Scientific Committee on Consumer Safety (SCCS) adopted SCCS/1459/11 - "Opinion on fragrance allergens in cosmetic products"; thus, it was concluded that people are sensitive to more substances than the 26 listed in Regulation 1223/2009. Approximately 56 more substances were planned to be added to the scope of the regulation, and in 2023, the new regulation came into effect: Commission Regulation (EC) 2023/1545 of 26 July 2023 amending Regulation (EC) No 1223/2009 of the European Parliament and of the Council and the Council Decision on the labelling of fragrance allergens in cosmetic products.
The additional substances listed in Annex III are as follows:
• 29 substances: Alpha-Terpinene, Terpinolene, Rose Ketones, 3-Propylidenephthalide, Methyl Salicylate, Acetyl Cedrene, Amyl Salicylate, Anethole, Benzaldehyde, Camphor, Beta-Caryophyllene, Carvone, Dimethyl Phenethyl Acetate, Hexadecanolactone, Hexamethylindanopyran, Linalyl Acetate, Menthol, Trimethylcyclopentenyl Methylisopentenol, Salicylaldehyd, Santalol, Sclareol, Terpineol, Tetramethyl acetyloctahydronaphthalenes, Trimethylbenzenepropanol, Vanillin, Eugenyl Acetate, Geranyl Acetate, Isoeugenyl Acetate, Pinene.
• 27 natural extracts and oils: Pinus Mugo, Pinus Pumila, Cedrus Atlantica Oil/Extract, Turpentine, Myroxylon Pereirae Oil/Extract, Lippia Citriodora Absolute, Cananga Odorata Oil/Extract, Cinnamomum Cassia Leaf Oil, Cinnamomum Zeylanicum Bark Oil, Citrus Aurantium Flower Oil, Citrus Aurantium Peel Oil, Citrus Aurantium Bergamia Peel Oil, Citrus Limon Peel Oil, Lemongrass Oil, Eucalyptus Globulus Oil, Eugenia Caryophyllus Oil, Jasmine Oil/Extract, Juniperus Virginiana Oil, Laurus Nobilis Leaf Oil, Lavandula Oil/Extract, Mentha Piperita Oil, Mentha Viridis Leaf Oil, Narcissus Extract, Pelargonium Graveolens Flower Oil, Pogostemon Cablin Oil, Rose Flower Oil/Extract, Santalum Album Oil.
These substances must be listed on cosmetic packaging according to the existing rules when present at the following or higher levels:
• 0.01% in rinsed-off cosmetics (e.g., soap, shower gel, shampoo),
• 0.001% in leave-on cosmetics (e.g., cream, lotion, tonic).
Labelling is mandatory if the substance exceeds the specified specific threshold concentrations, which are different for rinsed-off and leave-on products. Therefore, the new regulation expands this list to more than 80 allergens. It should be remembered that the purpose of this additional labelling is to inform sensitive individuals and let them know which components to avoid. Labelling is to tell them if the substance they are sensitive to is present in the product.
There is no need to change the formula and/or remove these substances, and/or consider reformulating these components. What is important is the expansion and update of the allergen list.
This situation has brought about new regulatory and implementation challenges such as:
• A large number of complex names for the same type of allergen for allergic consumers to memorize (56 new allergens),
• Space issues on labels due to very long ingredient lists.
To address these issues, a new approach to regulation was needed in the new regulation, allowing substances that cause the same cross-reactivity to be listed under a common group name instead of individual substance names in Annex III.
There are two regulatory approaches to naming allergens listed in Annex III in the regulation, referred to as 'Independent' and 'Grouped'. The 'Independent' allergen entry approach is the usual regulatory approach used for 24 allergens in the past. The 'Grouped' allergen entry approach is a new regulatory approach developed to solve the problem of long and complicated ingredient lists (especially for allergic individuals) that make labels too long and thus not consumer-friendly. Instead of forcing allergic individuals to memorize the full list of substances belonging to the same cross-reactivity group, only one name should be memorized for each group by relevant allergic consumers. For substances containing the same (type of) allergen, 'Group Names' are referred to as 'grouping names'.
For example, for the following three raw materials listed in line 354 of Annex III, the single name "Lemongrass Oil" should be indicated in the content as a group name.
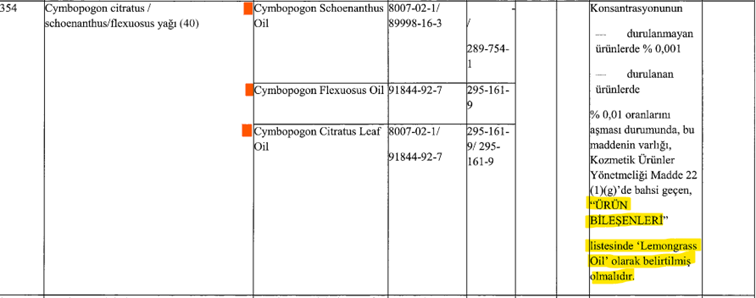
“If the concentration of the unreleased products exceeds 0.001% and the concentration of the released products exceeds 0.01%, the presence of this substance, as mentioned in Article (1)(g) of the Cosmetic Products Regulation, should be indicated in the ‘PRODUCT INGREDIENTS’ list as ‘Lemongrass Oil.’”
Regulation 2023/1545 confirms the transition period specified in the draft regulation we already know, and brand owners will need to adapt their labels to the new requirements according to the following timelines:
• Within 3 years for new cosmetic products; by 31 July 2026,
• Within 5 years for cosmetics already on the market; by 31 July 2028.
